It was 2:17 a.m. when the email hit my inbox.
Subject: [URGENT] 510(k) Submission Incomplete – Mechanism of Action Unclear
I’d spent 18 months building a smart catheter navigation system. $1.2M in seed funding. 37 all-nighters. My team’s hopes pinned on this moment. And the FDA’s verdict? “Your animation fails to demonstrate physiological interaction. Resubmit with clarified MoA.“
I stared at the screen, heart pounding like a metronome. Physiological interaction? We’d hired a “top” animation studio! They’d given us glossy 3D renders of the device… but zero context of how it moved through human tissue. To the FDA reviewer, it looked like a fancy toy, not a life-saving tool.
I grabbed my cold coffee. My hand shook. It spilled. All over my keyboard.
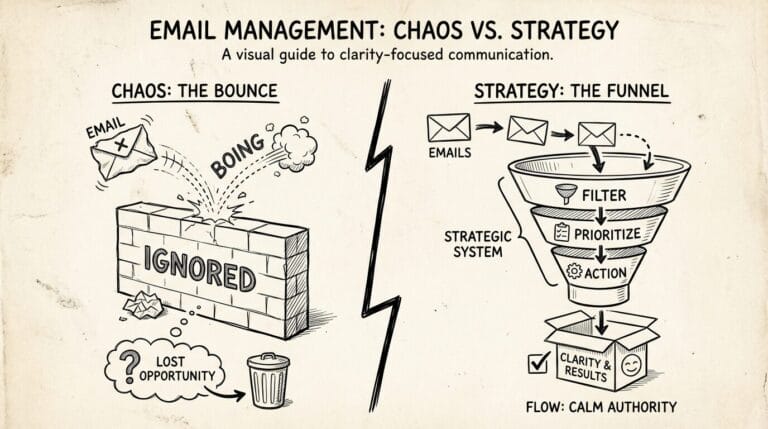
That’s when I realized: In Medtech, your animation isn’t just “marketing.” It’s your credibility. Your lifeline. Your ticket to saving lives. And if it fails, FDA animation compliance? You’re dead in the water.

I thought we were special. Turns out, we weren’t.
After that nightmare, I dug into FDA rejection logs. What I found shocked me:
FDA reviewers aren’t engineers. They’re clinicians scanning hundreds of submissions. If they can’t instantly see how your device interacts with the human body? Rejected.
We’d made the cardinal sin:
The result? Our submission got stuck in limbo for 5 months. Burned $87K in holding costs. Lost 3 hospital pilot deals.
Here’s the brutal part: Fixing it took less than 48 hours. Not because we rewrote our tech—but because we rewrote how we showed it.
Our new FDA animation compliance checklist had only 3 critical frames. No fluff. No “creative” extras. Just what the FDA actually needs to see:
What most people do wrong: Jumping straight into the device. What the FDA wants: Proof you understand the clinical problem
Our fix:
What most do wrong: Glowing 3D device spins with jargon What FDA wants: Clear cause-and-effect in human tissue
Our fix:
What most do wrong: “Our tech is amazing!” sales pitch What FDA wants: Measurable clinical outcome
Our fix:
The magic? We cut our animation from 2:15 to 0:38. We used real hospital footage (not stock art). Had a cardiologist narrate it (not a “voice talent”).

When we resubmitted, something wild happened.
The FDA reviewer replied in 72 hours with:
“Animation clearly demonstrates physiological interaction per 21 CFR 820.30. Submission accepted for review.”
Total turnaround: 17 days from resubmission to approval.
Our client (a nervous Medtech founder named David) called me sobbing:
“I showed this to my surgeon advisors. One said: ‘For the first time, I get how this saves lives.’ That’s when I knew we’d won.”
Why this works every time:
If your animation:
You’re risking rejection. And in Medtech? Every day delayed costs $29,000 in lost revenue (per MIT Medtech Report).

We’re not “animators.” We’re Medtech obsessives who’ve sat in FDA meetings. When you work with us:
Our FDA Animation Compliance Checklist:
Stop gambling with submissions. Stop paying studios who’ve never held a scalpel.
👉 Grab our FREE Medtech Guide → Scan Your Animation in 5 Minutes
Or skip the guesswork: Book a 15-minute FDA Animation Audit with our clinical team.
We’ll:
P.S. Last week, we fixed a neurotech client’s animation in 11 hours. Their FDA reply? “Approved with no questions.” Don’t wait 5 months to feel that relief. Let’s fix yours today.
Hi, I’m Ayan Wakil, the founder & CEO of Ayeans Studio.
Check out these and many other tips in our blog!



We provide services that successfully satisfy your Business Objectives
Ayeans Studio is a German-based Video Production Company, all set to deliver our pride services to US-based Clients