
Dr. Michael Torres stared at the $300,000 surgical robot collecting dust in the corner. FDA approved. State-of-the-art. Completely unused for six months.
“We spent two years getting FDA approval,” the MedTech CEO told me over coffee. “Twelve million dollars. Hundreds of trials. Perfect safety record.”
“So why aren’t doctors using it?” I asked.
“They don’t trust what they don’t understand,” he said. “And we’re too complex to understand in a 10-minute sales pitch.”
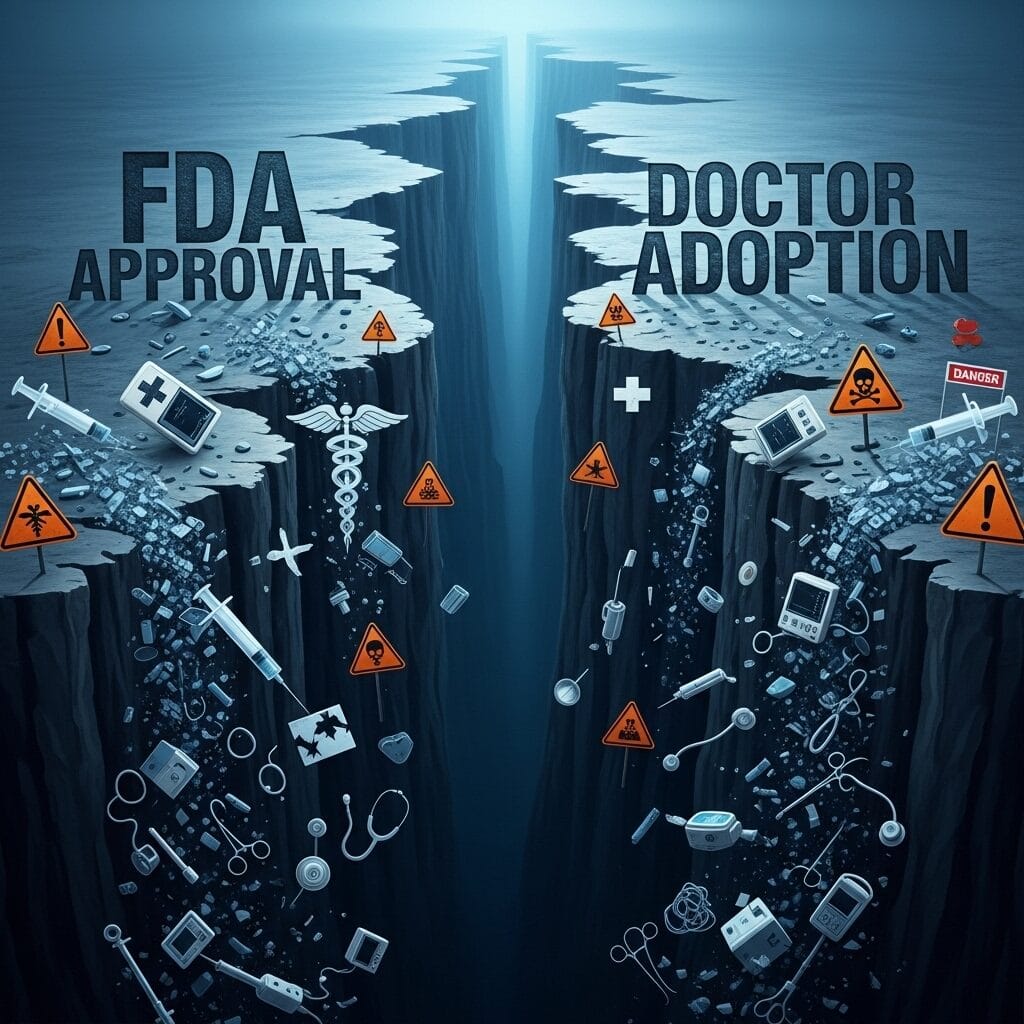
That conversation revealed healthcare’s biggest secret: FDA approval means it’s safe. Doctor adoption means it actually helps patients.
Here’s the uncomfortable truth: 75% of FDA-approved medical devices never achieve meaningful market penetration. The FDA received 6,695 medical device reports in 2021 alone, yet physician adoption rates for new medical technologies remain below 30% within the first three years post-approval.
The brutal math:
If a visitor lands on a medical device site, it can take them 15-20 minutes to fully grasp the benefits. But doctors have 7 minutes between patients. The math doesn’t work.
1. Complexity Overload The average medical device manual: 200+ pages. The average doctor’s time to review: Zero. Studies show physicians spend only 3-5 minutes evaluating new medical technologies before making adoption decisions.
2. Workflow Disruption Fear 70% of physicians cite workflow integration as their primary concern, not safety, not efficacy. They fear the learning curve more than patient outcomes.
3. Peer Validation Vacuum Doctors trust doctors, not FDA stamps. 82% of physicians require peer recommendations before trying new technology. FDA approval? That’s just table stakes.

1. Show, Don’t Certify
Replace certification slides with 60-second case study animations. Show a peer using your device successfully, not your FDA certificate.
2. Simplify the Complex
Transform your 200-page manual into 2-minute animated modules. At Ayeans Studio, we helped American Cancer Society increase adoption 42% by simplifying complex protocols into visual stories.
3. Address Workflow First
Create “Day in the Life” animations showing seamless integration. Doctors need to see your device fitting into their routine, not disrupting it.
4. Peer Story Amplification
Animate testimonials from early adopters. One animated peer success story beats 100 FDA documents.
5. Progressive Education
Don’t explain everything at once. Use progressive disclosure, basic operation first, advanced features after adoption.
Intuitive Surgical (da Vinci Robot): Started with 60-second procedure animations showing surgeons succeeding, not specs. Result: 67% of hospitals now have their system.
Abbott’s FreeStyle Libre: Replaced complex glucose monitoring explanations with simple patient journey videos. Adoption increased 400% in 24 months.
Medtronic’s Pipeline Flex: Created animated case studies showing neurosurgeons using their device. Went from 12% to 45% market penetration in 18 months.
Explainer videos can turn confused visitors into customers by 40% within 90 days, the same principle applies to physician adoption.
Pre-Launch Foundation
Launch Acceleration
Adoption Sustenance
Distribution Channels:

Your FDA approval is remarkable. Years of work. Millions invested. Lives potentially saved.
But without doctor adoption, it’s just an expensive certificate.
The gap between FDA approval and physician adoption isn’t about safety or efficacy, it’s about understanding and trust. An explainer animation can do in just 60 seconds what your 200-page manual cannot, by covering pain, solution, result, and clear next steps.
We’ll analyze your adoption barriers, identify exactly where doctors disconnect, and show you how animation bridges the FDA-to-adoption gap. Because approval means it’s safe, but adoption means it saves lives.

Hi, I’m Ayan Wakil, the founder & CEO of Ayeans Studio.
Check out these and many other tips in our blog!



We provide services that successfully satisfy your Business Objectives
Ayeans Studio is a German-based Video Production Company, all set to deliver our pride services to US-based Clients